Intra-Arterial Chemotherapy (IAC) for Retinoblastoma Made Simple
Sunday September 30, 2018
Intra-arterial chemotherapy (IAC) offers potential to save eyes and sight in children with retinoblastoma, when life is not at risk. Through the FAQ of this treatment, Sameh Soliman, MD reviews its goals, indications for use, benefits, risks and limitations, and offers resources for further reading.
Affiliation: Assistant professor, Department of Ophthalmology, University of Alexandria, Alexandria, Egypt; Clinical associate, Retinoblastoma program, the Hospital for Sick Children, Toronto, Ontario, Canada.
Retinoblastoma diagnosis bears a huge burden for the parents: the realization your child has a life threatening condition, an abundance of new information about genetics, treatments, and prognosis of retinoblastoma, and the possibility of increased lifelong risk of second cancers. This puts parents under severe stress and makes decision making about the best treatment very difficult.
Intra-arterial chemotherapy (IAC) is a recent treatment that multiple treatment centers advocate to avoid enucleation or systemic chemotherapy. In this article we aim to answer the most common parent questions about IAC to aid understanding.
Q1: What is IAC?
Intra-Arterial Chemotherapy (IAC, sometimes called ophthalmic artery chemosurgery – OAC) is a procedure for local delivery of the chemotherapeutic agent(s) directly into the ophthalmic artery supplying the eye, and consequently into the tumor.
Q2: What is the Principal of IAC?
The main goal is to increase the amount of chemotherapy delivered to the tumor, while avoiding the whole-body effects of systemic chemotherapy. This approach delivers chemotherapy to the retina, most tumors, the whole eye and sometimes the orbital tissue. The rest of the body gets a small dose, so side effects like hair loss and bone marrow suppression are reduced.
Q3: Is This Treatment Idea Recent?
In the 1990s, Japanese doctors treating retinoblastoma developed a technique that focused chemotherapy to the eye by inserting a balloon to re-direct blood flow in the internal carotid artery from the brain into the ophthalmic artery. In 2006, the New York group introduced direct cannulation of the ophthalmic artery to increase selectivity of the injected drug to the eye. Lately, many centers have adopted the technique.
Q4: How is the IAC Performed?
Under general anesthesia, the femoral artery (right or left leg) is identified in the child’s groin, and a catheter is introduced up the arterial system under x-ray visualization into the artery going to the brain (internal carotid artery). In the skull, the internal carotid artery curves and the ophthalmic artery is the first branch in this curve. A guide-wire helps direct the microcatheter into the opening of the ophthalmic artery. Drug injection begins after the position in the artery is verified.
Q5: Is Technical Success Guaranteed?
Many children have individual variations in anatomy. The curves and angle of the opening of the ophthalmic artery can make it technically difficult to cannulate the ophthalmic artery, and success is not guaranteed. The interventional radiologist can try assessing the artery from various arterial connections to reduce failure. Magnetic resonance angiography can help predict such anatomical variations.
Q6: What Are The Systemic Side Effects of Systemic Chemotherapy That Are Avoided In IAC?
Systemic chemotherapy in many centers needs the insertion of a central line for drug injection (Port-a-cath or Hickman line) that is later removed after tumor has stabilized and/or regressed. Systemic chemotherapy has short-term side-effects including nausea, vomiting, temporary hair loss, increased susceptibility to infections, increased probability of needing blood transfusion, and increased hospital admission with any fever. Long-term complications are hearing loss in 20% of children – that percentage decreases with older age (the highest frequency is in children less than 6 months), and low risk of leukemia.
Q7: What Are the Potential Side Effects and Complications of IAC?
Complications can be groin bruising (prevented by local pressure), arterial clotting (prevented by giving the child heparin), or stroke (theoretical risk but never reported). Eye complications include arterial blockage due to a clot (blood or drug particles), arterial spasm, temporary loss of the eye brows, temporary lid or ocular muscle weakness, orbital swelling or retinal detachment. Temporary or permanent loss of vision that may be partial or complete is possible. These complications are rare and have preventable strategies.
Q8: When Should IAC Be Used?
Indications vary according to the treatment center and its guidelines. In Canada, IAC is indicated as primary therapy for children with unilateral retinoblastoma classified as group B or C and some group D (new staging system TNMH cT2) when the optic nerve is seen and cancer poses no risk for spread beyond the eye, and there is potential for useful vision. IAC is also indicated as secondary therapy after failure of the primary therapy.
Bilateral simultaneous IAC is not recommended in Canada, and systemic chemotherapy is preferred when both eyes need chemotherapy. Bilateral simultaneous treatment increases the local acute risks, which are rare but devastating due to the potential for loss of sight. Systemic chemotherapy treats both eyes, has no toxicity to the eyes, and may also treat unrecognized extraocular cancer.
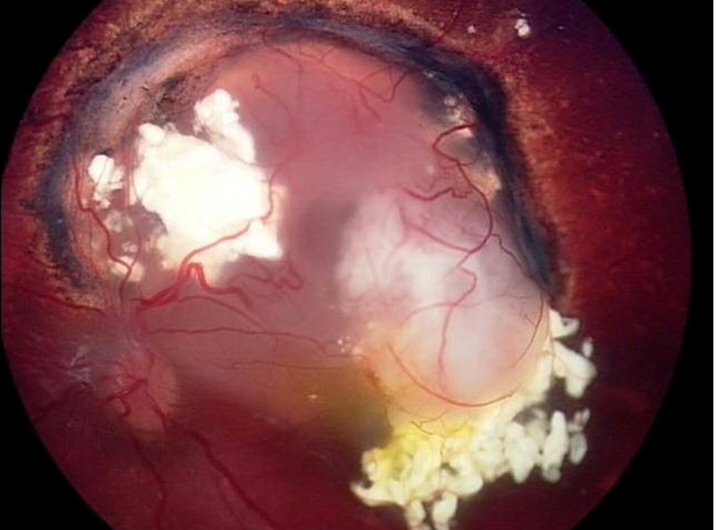
Recurrence after systemic chemotherapy.
The greyish fuzz arising from the white mass to the bottom right of the image is the recurrence.
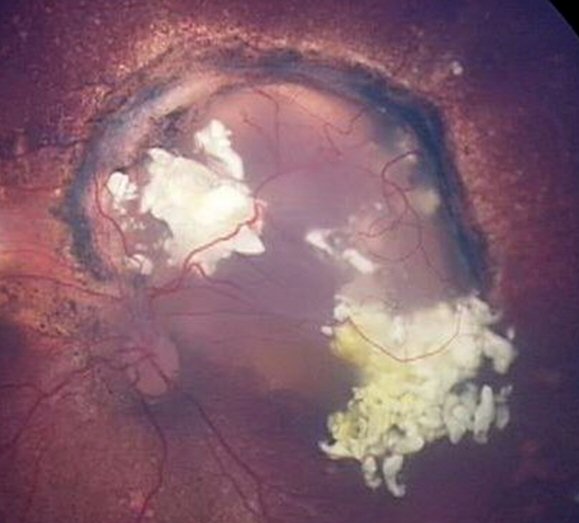
Regression of recurrence after 3 intra-arterial chemotherapy treatments. The tumour is inactive and will be lasered to ensure it is completely dead.
Q9: How Many Cycles Of IAC Are Given? And What Drugs?
Generally three cycles are given, three weeks apart. One or more drugs can be used, mainly Melphalan, topotecan and carboplatin. The dose is dependent on the child’s age and weight.
Q10: Is IAC Sufficient To Control The Tumor?
Chemotherapy aims to reduce tumor size so that focal tumor control can be definitively achieved using laser therapy, cryotherapy, plaque radiotherapy, intravitreal or periocular chemotherapy. Only occasionally does IAC control the tumor without additional focal therapy.
Q11: Can the Tumor Spread With IAC?
Metastasis is a major concern, but not because IAC itself spreads tumor. Rather, IAC treats only the eye, not any tumor cells that might have escaped from the eye. In comparison, systemic chemotherapy has a good chance to destroy escaped tumor cells before they are detected. Depending on the clinical cancer stage for the eye, there is a 2% to 15% risk of tumor cells escaping from the eye. Delay in diagnosis and starting treatment increases this risk.
Q12: Are There Any Published Studies Discussing Outcomes Of IAC?
Many publications have addressed the effectiveness of IAC. However, most are retrospective reviews of children previously treated, or prospective studies of children in treatment, without a comparative group, which undermines the conclusions. This is a major problem in treating retinoblastoma, since very few proper clinical trials have been conducted. Few publications addressed the comparison between IAC, enucleation and systemic chemotherapy in historical cohorts. The role of IAC for retinoblastoma is not yet clear.
Q13: What Are The Limitations Of IAC?
The main limitation of IAC is that it is less effective in group E eyes, and dangerous to hide spread of tumor when the optic nerve cannot be seen.
Cost is also very high – estimated around 310,000-430,000 USD per 6 cycles, depending on whether one or both eyes are being treated. IAC is the most expensive treatment for retinoblastoma to date.
Q14: Do You Recommend IAC? Are There Any Contraindications?
We recommend IAC when clinical exam and imaging shows it is safe for the child – as described in Q8.
We do not recommend IAC for international patients due to the huge cost and potential for poor follow up.
Bilateral simultaneous treatment expands the local acute risks which are rare but devastating if they occur due to the risk for permanent loss of sight.
Unilateral group E with potential for imminent extraocular disease, no visible optic nerve, and poor visual potential is a strong contraindication, since it may put the child’s life at risk.
Q15: How Can I Identify Where To Have IAC?
You can access the One Retinoblastoma World map to identify your nearest Retinoblastoma Center for IAC. Your Retinoblastoma team of doctors can advise if IAC can contribute safely to the care of your child.
Q16: Do You Have Any Final Recommendation?
Consider your child as a whole, your budget and the potential outcome regarding life and vision before taking your final decision on which treatment is best. Most important, ask your child’s doctors all the questions you can think of, until you feel you are fully informed to make the best possible choices for your child.
Q17 What Can I Read About This Treatment?
You may find the following references valuable:
- Dimaras H, Corson TW, Cobrinik D, et al. Retinoblastoma. Nat Rev Dis Primers. 2015;1:15021. (full text)
- Dimaras H, Kimani K, Dimba EA, et al. Lancet. 2012;379(9824):1436-1446. (full text)
- Yousef YA, Soliman SE, Astudillo PP, et al. Intra-arterial Chemotherapy for Retinoblastoma: A Systematic Review. JAMA ophthalmology. 2016;134(6):584-591.
- Abramson DH. Chemosurgery for retinoblastoma: what we know after 5 years. Arch Ophthalmol. 2011;129(11):1492-1494.
- National Retinoblastoma Strategy Canadian Guidelines for Care / Stratégie thérapeutique du rétinoblastome guide clinique canadien. Can J Ophthalmol. 2009;44(Supp 2):S1-88. (Full test PDF)
- Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129(6):732-737.
- Soliman SE, Gallie BL, Shaikh F. Intra-arterial Chemotherapy for Retinoblastoma-Reply. JAMA ophthalmology. 2016;134(10):1203.
- Soliman SE, Dimaras H, Gallie B, Shaikh F. Re: Metastatic deaths in retinoblastoma patients treated with intraarterial chemotherapy (ophthalmic artery chemosurgery) worldwide. Int J Retina Vitreous. 2018;4:19.
- Munier FL, Mosimann P, Puccinelli F, et al. First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment. Br J Ophthalmol. 2016. (Full text)
- Aziz HA, Lasenna CE, Vigoda M, et al. Retinoblastoma treatment burden and economic cost: impact of age at diagnosis and selection of primary therapy. Clin Ophthalmol. 2012;6:1601-1606. (Full text)
About the Author
Sameh Soliman is a pediatric ophthalmologist focusing on retinoblastoma. He trained in Alexandria, Egypt and Toronto, Canada. He became the first retinoblastoma specialist in Alexandria, where he instigated the first ocular oncology clinic and set up retinoblastoma management protocols. He is based in the Hospital for Sick Children, Toronto, Canada under the mentorship of Dr. Brenda Gallie. He has contributed to development of intravitreal chemotherapy with Dr. Francis Munier, and prenatal management of familial retinoblastoma and OCT guided therapy with Dr. Gallie.




Share this entry